Seasonal Influenza Vaccines Therapeutics Market Poised for 136% Growth by 2035, Empowering Manufacturers
Rising demand, advanced production, and strategic partnerships drive a projected USD 24.1B market by 2035.
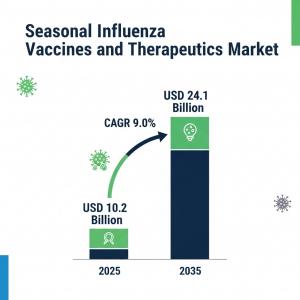
NEW YORK, DE, UNITED STATES, August 14, 2025 /EINPresswire.com/ -- The Seasonal Influenza Vaccines Therapeutics Market is entering a decade of unprecedented expansion. Valued at USD 10.2 billion in 2025, the market is forecast to reach USD 24.1 billion by 2035, reflecting a compound annual growth rate (CAGR) of 9.0%. This trajectory underscores the sector’s growing role in addressing evolving viral threats while opening up scalable opportunities for vaccine manufacturers.
Manufacturers are positioned at the heart of this growth, with heightened demand being fueled by the convergence of advanced vaccine technologies, strengthened immunization programs, and global awareness of influenza’s impact. The stakes are higher than ever as seasonal outbreaks, coupled with unpredictable viral mutations, demand faster, broader, and more efficient vaccine solutions.
Quadrivalent Vaccines Lead Market Evolution
In 2025, quadrivalent vaccines are projected to dominate the market with a 64.5% revenue share. This leadership stems from their enhanced coverage, targeting four virus strains instead of three—significantly reducing the risk of mismatches during influenza season.
From a manufacturing standpoint, the ability to produce these broader-spectrum vaccines at scale without substantial cost increases has been a key driver. The alignment between epidemiological needs and production capabilities has made quadrivalent vaccines the preferred choice for healthcare providers and policymakers alike.
For manufacturers, this segment represents a stable, high-demand product line with strong government and institutional support, ensuring predictable procurement cycles and long-term contracts.
Click Here for More Information:- https://www.futuremarketinsights.com/reports/seasonal-influenza-vaccines-therapeutics-market
Adult Immunization Remains the Prime Demand Driver
The adult population is expected to account for 71.0% of market revenue in 2025. This segment’s resilience is supported by the heightened susceptibility of adults—particularly older individuals and those with chronic illnesses—to flu-related complications.
Public health campaigns targeting workforce productivity, caregiver health, and hospital burden reduction have intensified adult vaccination rates. In many regions, workplace and pharmacy-based immunization programs have streamlined access, creating steady demand patterns manufacturers can plan around.
This demographic’s consistent uptake offers production stability, allowing manufacturers to forecast volumes with greater precision and invest confidently in facility upgrades or expansion.
Injections Maintain Stronghold as the Preferred Delivery Route
Injections are set to account for 88.0% of market revenue in 2025, reinforcing their position as the gold standard in vaccine administration. Manufacturers benefit from the entrenched infrastructure for injectable vaccines, ensuring high compatibility with existing distribution and delivery networks.
Advances in needle technology and vaccine formulation stability have further cemented this route’s dominance, reducing patient hesitation and improving adherence to vaccination schedules. For producers, the established logistics of injectable vaccine manufacturing mean fewer disruptive transitions and sustained cost-efficiency.
Government Initiatives and Technological Breakthroughs Accelerate Market Momentum
Governments worldwide are actively funding influenza vaccination programs, integrating them into national immunization schedules, and investing in surveillance systems to track viral changes. This coordinated approach ensures not only higher vaccination rates but also predictable procurement, giving manufacturers greater certainty in planning production runs.
Innovation remains a cornerstone of growth. In August 2024, Seqirus advanced its self-amplifying mRNA influenza vaccine technology—a development that highlights the sector’s shift toward faster, more adaptable production methods. The U.S. Centers for Disease Control and Prevention (CDC) has reiterated the necessity of evolving vaccines in step with seasonal virus mutations, reinforcing the need for agile manufacturing capabilities.
Regional Growth Pathways for Manufacturers
North America currently leads the global market, supported by advanced healthcare infrastructure, robust public awareness, and strong government vaccination policies. The region’s mature regulatory environment and funding for innovation make it a fertile ground for manufacturers to launch next-generation vaccine platforms.
Europe follows as a key growth hub, driven by an aging population, high flu incidence rates, and demand for novel vaccine formulations. Manufacturers with the capacity to navigate stringent European regulations stand to gain a strong foothold in this competitive landscape.
Asia-Pacific is rapidly emerging as a high-growth opportunity, with rising influenza prevalence, expanding healthcare infrastructure, and increasing government support for immunization. Global vaccine leaders are already investing heavily in local partnerships and manufacturing facilities to capture market share in this region.
Strategic Imperatives for Manufacturers
For vaccine producers, the coming decade offers both opportunity and complexity. To capitalize on the market’s projected growth, manufacturers must focus on:
• Scaling production of quadrivalent and advanced vaccine types to meet evolving epidemiological needs.
• Leveraging regional demand patterns to optimize distribution networks and reduce time-to-market.
• Investing in R&D for broader-spectrum and longer-lasting vaccines to remain competitive against emerging strains.
• Building collaborative partnerships with governments and healthcare organizations to secure long-term supply agreements.
By aligning manufacturing strategies with these market drivers, producers can position themselves not just as suppliers, but as strategic partners in global public health resilience.
Get Sample Report: - https://www.futuremarketinsights.com/reports/sample/rep-gb-2498
Future Outlook: Resilient, Responsive, and Ready
The Seasonal Influenza Vaccines Therapeutics Market’s projected growth to USD 24.1 billion by 2035 reflects a sustained commitment to tackling one of the most persistent global health challenges. For manufacturers, this is a pivotal moment to invest in capacity, technology, and partnerships that will define competitive advantage for years to come.
With innovation driving efficiency and demand surging across developed and emerging regions, the sector’s future promises not just financial returns but a meaningful contribution to global health security. Those who adapt swiftly to technological advances and evolving demand will be best placed to lead this next era of vaccine manufacturing.
Editor’s Note: This release is intended for informational purposes only and should not be construed as financial or medical advice.
Discover Related Research:-
Urinary Tract Infection (UTI) Treatment Market
https://www.futuremarketinsights.com/reports/urinary-tract-infections-market
Ulcerative Colitis Treatment Market
https://www.futuremarketinsights.com/reports/ulcerative-colitis-treatment-market
Glaucoma Therapeutics Market
https://www.futuremarketinsights.com/reports/glaucoma-therapeutics-market
Rahul Singh
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.