Pulmonary Hypertension associated with Interstitial Lung Disease Market to Witness Upsurge in Growth During the Forecast Period (2025–2034) | DelveInsight
The PH-ILD market is driven by increased disease recognition, improved diagnostic techniques, and a growing ILD patient base. Demand is further supported by the approval of inhaled therapies like TYVASO and the ongoing development of targeted treatments addressing the unmet need in functional improvement and disease progression.
New York, USA, Aug. 14, 2025 (GLOBE NEWSWIRE) -- Pulmonary Hypertension associated with Interstitial Lung Disease Market to Witness Upsurge in Growth During the Forecast Period (2025–2034) | DelveInsight
The PH-ILD market is driven by increased disease recognition, improved diagnostic techniques, and a growing ILD patient base. Demand is further supported by the approval of inhaled therapies like TYVASO and the ongoing development of targeted treatments addressing the unmet need in functional improvement and disease progression.
DelveInsight’s Pulmonary Hypertension associated with Interstitial Lung Disease Market Insights report includes a comprehensive understanding of current treatment practices, emerging PH-ILD drugs, market share of individual therapies, and current and forecasted PH-ILD market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Pulmonary Hypertension associated with Interstitial Lung Disease Market Report
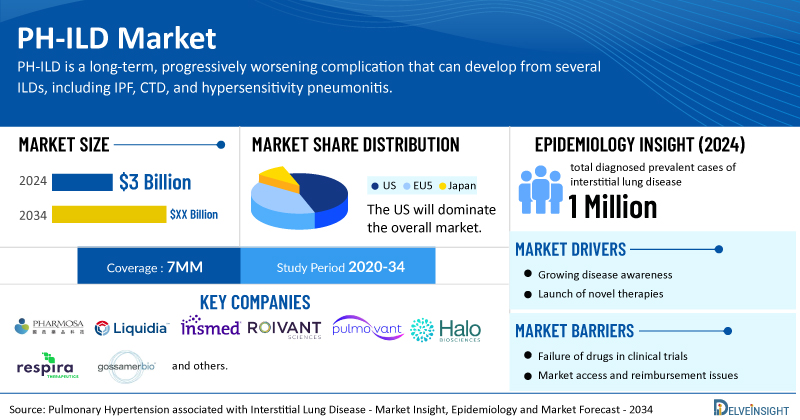
- According to DelveInsight’s analysis, the market size for PH-ILD was found to be USD 3 billion in the 7MM in 2024.
- The United States accounts for the largest market size of PH-ILD, in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan.
- DelveInsight research indicates that in 2024, there were more than 1 million total diagnosed prevalent cases of interstitial lung disease across the 7MM, which are expected to increase by 2034.
- Prominent companies, including Pharmosa BioPharm, Liquidia Corporation, Insmed, Roivant Sciences, Pulmovant, Halo Biosciences, Respira Therapeutics, Gossamer Bio, and others, are actively working on innovative PH-ILD drugs.
- Some of the key PH-ILD therapies in the pipeline include Treprostinil liposomal, treprostinil palmitil inhalation powder, mosliciguat, H1614, Vardenafil (RT234), Seralutinib, and others. These novel PH-ILD therapies are anticipated to enter the PH-ILD market in the forecast period and are expected to change the market.
Discover which PH-ILD medications are expected to grab the market share @ Pulmonary Hypertension associated with Interstitial Lung Disease Market Report
Pulmonary Hypertension associated with Interstitial Lung Disease Market Dynamics
The PH-ILD market dynamics are anticipated to change in the coming years. Advancements in imaging, such as echocardiography, HRCT, and cardiac MRI, are improving early detection of pulmonary vascular changes in PH-ILD, enabling timely diagnosis and informed management, while innovations like inhalation-based drug delivery enhance lung-targeted bioavailability and reduce systemic side effects; coupled with the limited number of approved therapies, these factors create strong innovation potential, with anticipated regulatory approvals of emerging treatments expected to expand options and drive market growth in this underserved clinical segment.
Furthermore, many potential therapies are being investigated for the treatment of PPH-ILD, and it is safe to predict that the treatment space will significantly impact the PH-ILD market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the PH-ILD market in the 7MM.
However, several factors may impede the growth of the PH-ILD market. The presence of overlapping symptoms such as breathlessness, fatigue, and reduced exercise capacity makes PH in patients with ILD difficult to distinguish, and the absence of strong biomarkers further delays accurate diagnosis; combined with the limited number of pipeline drugs, reimbursement challenges, and intensifying market competition, these factors collectively hinder innovation, slow the development of effective treatments, and potentially restrict patient access to novel PH-ILD therapies.
Moreover, PH-ILD treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the PH-ILD market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the PH-ILD market growth.
Pulmonary Hypertension associated with Interstitial Lung Disease Treatment Market
The therapeutic landscape for PH-ILD is experiencing a significant transformation, fueled by greater disease awareness, advancements in imaging and diagnostic tools, and the introduction of inhaled therapies designed to target the pulmonary vasculature. Although PH-ILD remains a progressive and life-threatening condition, the market is shifting toward innovative treatments that aim to improve functional capacity and slow disease progression. Historically, the pharmacological options for PH-ILD have been limited, with most therapies adapted from PAH management.
PAH-specific agents, such as PDE5 inhibitors like sildenafil, have been frequently used off-label for their pulmonary vasodilatory effects and generally favorable safety profile. Endothelin receptor antagonists (ERAs), including ambrisentan and bosentan, have also been prescribed, albeit cautiously, due to potential risks of worsening oxygenation in ILD patients. Prostacyclin analogs and receptor agonists such as treprostinil and iloprost are available in both inhaled and parenteral formulations; however, systemic administration can be constrained by adverse effects and oxygenation issues, underscoring the value of inhaled delivery methods like TYVASO, which provide targeted pulmonary vasodilation while limiting systemic exposure.
In May 2025, the US FDA approved YUTREPIA (treprostinil) inhalation powder for improving exercise capacity in adults with PAH and PH-ILD. Leveraging Liquidia’s PRINT technology, it delivers precise, uniform particles deep into the lungs. As the first dry powder prostacyclin, YUTREPIA offers enhanced ease of use and potential adherence advantages through a low-resistance, breath-actuated inhalation device.
Learn more about the PH-ILD treatment options @ Pulmonary Hypertension associated with Interstitial Lung Disease Treatment Guidelines
Pulmonary Hypertension associated with Interstitial Lung Disease Emerging Drugs and Companies
Key drugs currently in the PH-ILD pipeline include treprostinil liposomal (Pharmosa BioPharm/ Liquidia Corporation), treprostinil palmitil inhalation powder (Insmed), mosliciguat (Roivant Sciences/Pulmovant), H1614 (HB-1614) (Halo Biosciences), and among others—reflecting diverse mechanisms aimed at improving treatment outcomes.
Liposomal treprostinil, administered twice daily via a smart nebulizer, is engineered for sustained pulmonary delivery with lower peak plasma concentrations and reduced upper airway irritation. Co-developed by Pharmosa BioPharm and Liquidia Corporation, it has been highlighted as a leading candidate in attribute analyses and is currently in Phase III trials for PH-ILD.
TPIP, developed by Insmed, is a novel orally inhaled prostacyclin agonist in Phase II/III studies. Interim results from a Phase II trial in 31 PH-ILD patients indicated promising efficacy, and Insmed plans to initiate Phase III in the second half of 2025. In June 2025, TPIP also demonstrated positive Phase IIb results in PAH, with a 35% reduction in pulmonary vascular resistance (PVR) and improvements in 6-minute walk distance and NT-proBNP. The company intends to discuss Phase III plans with the FDA and launch a PH-ILD trial by year-end.
Seralutinib, an inhaled tyrosine kinase inhibitor targeting PDGFR, CSF1R, and c-KIT, is being developed by Gossamer Bio (with XOMA holding royalty interests) to address pulmonary vascular remodeling. The Phase II TORREY trial in 86 PAH patients supports advancement to a Phase III PH-ILD study, scheduled to start in Q4 2025. In May 2024, Gossamer Bio partnered with Chiesi Group under a global licensing agreement to accelerate development and commercialization for PAH and PH-ILD.
HB-1614 (H1614 or H01), Halo Biosciences’ lead program, is a novel reformulation of 4-methylumbelliferone designed to inhibit hyaluronan synthesis—an important driver of chronic inflammation and fibrosis in the extracellular matrix (ECM). Clinical trials are expected to begin in 2026. In June 2025, Halo Biosciences announced the publication of Phase IIa SATURN study results in Thorax, marking a key milestone in developing HB-1614 for PH-ILD.
The anticipated launch of these emerging PH-ILD therapies are poised to transform the PH-ILD market landscape in the coming years. As these cutting-edge PH-ILD therapies continue to mature and gain regulatory approval, they are expected to reshape the PH-ILD market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for PH-ILD, visit @ Pulmonary Hypertension associated with Interstitial Lung Disease Management
Recent Developments in the Pulmonary Hypertension associated with Interstitial Lung Disease Market
- In June 2025, Insmed reported strong Phase IIb results for TPIP as a once-daily PAH treatment, showing a 35% placebo-adjusted PVR reduction, improved 6-minute walk distance, and a 60% NT-proBNP decline.
- In May 2025, YUTREPIA (treprostinil) inhalation powder received full FDA approval for improving exercise capacity in adults with PAH and PH-ILD. As the first dry powder prostacyclin therapy enabled by Liquidia’s PRINT technology, YUTREPIA delivers uniform particles to the deep lung via a low-resistance, breath-actuated device, offering enhanced usability and potential adherence benefits.
- In May 2025, Gossamer Bio reported the completion of enrollment for the ongoing global Phase III PROSERA study, which is evaluating seralutinib in patients with Functional Class II and III PAH
Pulmonary Hypertension associated with Interstitial Lung Disease Overview
PH-ILD is a long-term, progressively worsening complication that can develop from several interstitial lung diseases (ILDs), including idiopathic pulmonary fibrosis (IPF), connective tissue disease (CTD), and hypersensitivity pneumonitis. It results from increased pressure in the pulmonary arteries caused by lung scarring and structural changes in the blood vessels, placing considerable strain on both the heart and lungs. Common symptoms include increasing shortness of breath, fatigue, and reduced exercise tolerance, with the condition gradually impairing daily activities and quality of life. Without prompt treatment, PH-ILD can advance to right heart failure and is associated with significant morbidity and mortality. As one of the most severe ILD-related complications, it underscores the importance of early detection, proactive screening in high-risk groups, and the development of targeted, more effective therapies.
Diagnosis is often difficult because PH-ILD symptoms overlap with those of the underlying ILD. Clinicians should suspect PH-ILD in ILD patients showing unexplained worsening of breathlessness or exercise limitation. Diagnostic approaches include echocardiography for initial evaluation and pulmonary function tests revealing a disproportionate drop in the Diffusing Capacity of the Lung for Carbon Monoxide (DLCO). High-resolution CT scans help assess the extent of lung fibrosis, while right heart catheterization remains the definitive method for confirming pulmonary hypertension. Early and accurate identification through regular monitoring of high-risk ILD patients is key to initiating timely treatment and improving prognosis.
Pulmonary Hypertension associated with Interstitial Lung Disease Epidemiology Segmentation
The PH-ILD epidemiology section provides insights into the historical and current PH-ILD patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The PH-ILD market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Prevalent Cases of ILD
- Type-specific Diagnosed Prevalent Cases of ILD
- Type-specific Diagnosed Prevalent Cases of PH-ILD
- Total Diagnosed Prevalent Cases of PH-ILD
- Gender-specific Diagnosed Prevalent Cases of PH-ILD
Download the report to understand which factors are driving PH-ILD epidemiology trends @ Pulmonary Hypertension associated with Interstitial Lung Disease Treatment Algorithm
| Pulmonary Hypertension associated with Interstitial Lung Disease Report Metrics | Details |
| Study Period | 2020–2034 |
| Pulmonary Hypertension associated with Interstitial Lung Disease Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Pulmonary Hypertension associated with Interstitial Lung Disease Market Size in 2024 | USD 3 Billion |
| Key Pulmonary Hypertension associated with Interstitial Lung Disease Companies | Pharmosa BioPharm, Liquidia Corporation, Insmed, Roivant Sciences, Pulmovant, Halo Biosciences, Respira Therapeutics, Gossamer Bio, and others |
| Key Pulmonary Hypertension associated with Interstitial Lung Disease Therapies | Treprostinil liposomal, treprostinil palmitil inhalation powder, mosliciguat, H1614, Vardenafil (RT234), Seralutinib, and others |
Scope of the Pulmonary Hypertension associated with Interstitial Lung Disease Market Report
- Pulmonary Hypertension associated with Interstitial Lung Disease Therapeutic Assessment: Pulmonary Hypertension associated with Interstitial Lung Disease current marketed and emerging therapies
- Pulmonary Hypertension associated with Interstitial Lung Disease Market Dynamics: Conjoint Analysis of Emerging Pulmonary Hypertension associated with Interstitial Lung Disease Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Pulmonary Hypertension associated with Interstitial Lung Disease Market Access and Reimbursement
Discover more about Pulmonary Hypertension associated with Interstitial Lung Disease drugs in development @ Pulmonary Hypertension associated with Interstitial Lung Disease Clinical Trials
Table of Contents
| 1 | Key Insights |
| 2 | Report Introduction |
| 3 | PH-ILD Market Overview at a Glance |
| 3.1 | Market Share (%) Distribution of PH-ILD by Therapies in the 7MM in 2024 |
| 3.2 | Market Share (%) Distribution of PH-ILD by Therapies in the 7MM in 2034 |
| 4 | Executive Summary |
| 5 | Key Events |
| 6 | Disease Background and Overview |
| 6.1 | Introduction |
| 6.2 | Classification |
| 6.3 | Clinical Manifestations |
| 6.4 | Risk Factors |
| 6.5 | Pathogenesis |
| 6.6 | Recurrent PH-ILD |
| 6.7 | Biomarkers |
| 6.8 | Diagnosis |
| 6.8.1 | Differential Diagnosis |
| 6.8.2 | Diagnostic Algorithm |
| 6.8.3 | Diagnostic Guidelines and Recommendations |
| 6.9 | Treatment and Management |
| 6.9.1 | Treatment Algorithm |
| 6.9.2 | Treatment Guidelines and Recommendations |
| 7 | Methodology |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumptions and Rationale: The 7MM |
| 8.2.1 | Total Diagnosed Prevalent Cases of ILD |
| 8.2.2 | Type-specific Diagnosed Prevalent Cases of ILD |
| 8.2.3 | Type-specific Diagnosed Prevalent Cases of PH-ILD |
| 8.2.4 | Total Diagnosed Prevalent Cases of PH-ILD |
| 8.2.5 | Gender-specific Diagnosed Prevalent Cases of PH-ILD |
| 8.3 | Total Diagnosed Prevalent Cases of PH-ILD in the 7MM |
| 8.4 | The US |
| 8.4.1 | Total Diagnosed Prevalent Cases of ILD |
| 8.4.2 | Type-specific Diagnosed Prevalent Cases of ILD |
| 8.4.3 | Type-specific Diagnosed Prevalent Cases of PH-ILD |
| 8.4.4 | Total Diagnosed Prevalent Cases of PH-ILD |
| 8.4.5 | Gender-specific Diagnosed Prevalent Cases of PH-ILD |
| 8.5 | EU4 and the UK |
| 8.6 | Japan |
| 9 | Patient Journey |
| 10 | Marketed Drugs |
| 10.1 | Key Cross Competition |
| 10.2 | YUTREPIA (Treprostinil): Liquidia Corporation |
| 10.2.1 | Product Description |
| 10.2.2 | Regulatory Milestones |
| 10.2.3 | Other Developmental Activities |
| 10.2.4 | Clinical Trials Information |
| 10.2.5 | Safety and Efficacy |
| 10.3 | TYVASO/TYVASO DPI/ TREPROST (Treprostinil): United Therapeutics |
| List to be continued in the final report. | |
| 11 | Emerging Drugs |
| 11.1 | Key Cross Competition |
| 11.2 | Treprostinil liposomal (L606): Pharmosa BioPharm/ Liquidia Corporation |
| 11.2.1 | Drug Description |
| 11.2.2 | Other Developmental Activities |
| 11.2.3 | Clinical Trials Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analysts’ View |
| 11.3 | Treprostinil Palmitil Inhalation Powder (TPIP): Insmed |
| 11.4 | Mosliciguat: Roivant Sciences/Pulmovant |
| List to be continued in the final report. | |
| 12 | PH-ILD – 7MM Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Key Market Forecast Assumptions |
| 12.2.1 | Cost Assumptions and Rebates |
| 12.2.2 | Pricing Trends |
| 12.2.3 | Analogue Assessment |
| 12.2.4 | Launch Year and Therapy Uptake |
| 12.3 | Market Outlook |
| 12.4 | Attribute Analysis |
| 12.5 | Total Market Size of PH-ILD in the 7MM |
| 12.6 | Market Size of PH-ILD by Therapies in the 7MM |
| 12.7 | Market Size of PH-ILD in the United States |
| 12.7.1 | Total Market Size of PH-ILD |
| 12.7.2 | Market Size of PH-ILD by Therapies in the United States |
| 12.8 | Market Size of PH-ILD in EU4 and the UK |
| 12.9 | Market Size of PH-ILD in Japan |
| 13 | KOL Views |
| 14 | Unmet Needs |
| 15 | SWOT Analysis |
| 16 | Market Access and Reimbursement |
| 16.1 | The United States |
| 16.2 | In EU4 and the UK |
| 16.3 | Japan |
| 17 | Bibliography |
| 18 | Report Methodology |
Related Reports
Interstitial Lung Disease Market
Interstitial Lung Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key ILD companies, including Roche, aTyr Pharma, Boehringer Ingelheim, FibroGen, LTT Bio-Pharma, Bristol-Myers Squibb, Prometheus Biosciences, HEC Pharm, Bayer, Insmed, Avalyn Pharma, PureTech Health, Novartis, Horizon, MediciNova, Endeavor BioMedicines, Pliant Therapeutics, Kadmon Pharmaceuticals, GenKyoTex, Lung Therapeutics, AdAlta, Ark Biosciences, among others.
Interstitial Lung Disease Pipeline
Interstitial Lung Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key interstitial lung disease companies, including AdAlta, Bristol-Myers Squibb, aTyr Pharma, Avalyn Pharmaceuticals, Beijing Continent Pharmaceutical, Regend Therapeutics, Reata Pharmaceuticals, FibroGen, PureTech Health, Bellerophon Pulse Technologies, OncoArendi Therapeutics, LTT Bio-Pharma, EmphyCorp, Genentech, Cudetaxestat, Boehringer Ingelheim, Prometheus Biosciences, HEC Pharm, Bayer, Insmed, Bristol-Myers Squibb, Avalyn Pharma, PureTech Health, Roche, Ark Biosciences, Novartis, Lung Therapeutics, Horizon, MediciNova, Endeavor BioMedicines, Pliant Therapeutics, Kadmon Pharmaceuticals, GenKyoTex, Taiho Pharmaceutical, Syndax Pharmaceuticals, Metagone Biotech, Galecto Biotech, CSL Behring and AstraZeneca, among others.
Pulmonary Hypertension Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key pulmonary hypertension companies, including Tenax Therapeutics, Inc., Tectonic Therapeutic, OrphAI Therapeutics, Pharmosa Biopharm Inc., United Therapeutics, Pfizer, Attgeno AB, Cumberland Pharmaceuticals, Pulmovant, Inc., Guangzhou Magpie Pharmaceuticals Co., Ltd., Foresee Pharmaceuticals Co., Ltd., Novartis Pharmaceuticals, ABLi Therapeutics, Inc., 35Pharma Inc., Beijing Continent Pharmaceutical Co, Ltd., Keros Therapeutics, Inc., Bayer, AstraZeneca, Apollo Therapeutics Ltd, among others.
Pulmonary Hypertension Pipeline
Pulmonary Hypertension Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key pulmonary hypertension companies, including Tenax Therapeutics, Inc., Tectonic Therapeutic, OrphAI Therapeutics, Pharmosa Biopharm Inc., United Therapeutics, Pfizer, Attgeno AB, Cumberland Pharmaceuticals, Pulmovant, Inc., Guangzhou Magpie Pharmaceuticals Co., Ltd., Foresee Pharmaceuticals Co., Ltd., Novartis Pharmaceuticals, ABLi Therapeutics, Inc., 35Pharma Inc., Beijing Continent Pharmaceutical Co, Ltd., Keros Therapeutics, Inc., Bayer, AstraZeneca, Apollo Therapeutics Ltd, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
